Metabolic monitoring usingthe CWE Gemini O2 and CO2 monitor | ||
| ||
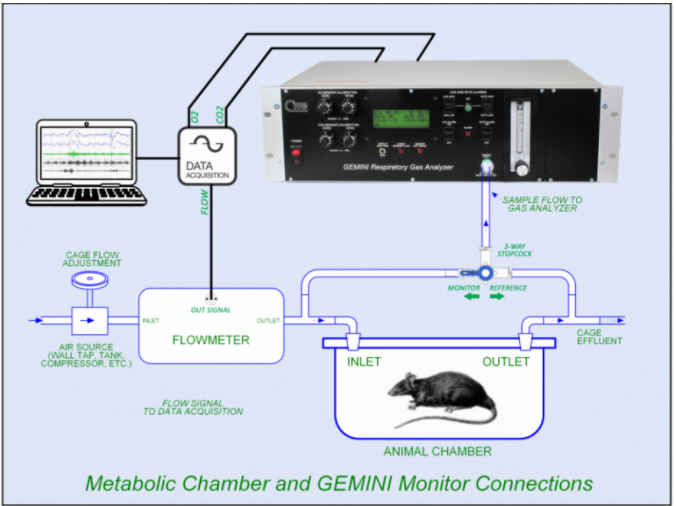
Introduction There is a long history of using indirect calorimetry to measure energy expenditure, oxygen consumption (VO2), and carbon dioxide production (VCO2) in laboratory animals. Large animals, including humans, can most conveniently be monitored using a fitted face mask with attached pneumotach for flow measurement and a sample port for the gas analyzer. For smaller animals such as rodents, this approach is difficult or impossible to implement successfully, and metabolic chambers are used instead. A metabolic chamber can be any sealed enclosure with one inlet and one outlet (see Figure). Fresh air (Vi) flows through the chamber at a known flow rate, so the animal inspires from this flow and expires into it. Because the animal is both consuming O2 and producing CO2 through metabolic activity, the chamber outlet gas will have a reduced O2 fraction (FoO2) and an increased CO2 fraction (FoCO2). The flow rate (Vi) through the chamber is adjusted so that the CO2 in the chamber does not build up excessively, and the O2 is not depleted, but still allowing measurable input/output differentials for gas measurements. In practice, a 0.2 – 1.0% FoCO2/FiCO2 differential is adequate. The chamber flow (Vi) is adjusted until the FoCO2 falls into this range. These measurements: Vi, FiO2, FiCO2, FoO2 and FoCO2 can then be manipulated to calculate oxygen consumption (VO2), CO2 production (VCO2), Respiratory Exchange Ratio (RER), and metabolic heat production or energy expenditure (EE). This flow-through system is known as open-circuit calorimetry. Experimental setup The Figure shows the basic equipment setup. A controlled flow (Vi) is monitored by the flowmeter. A gas analyzer measures O2 and CO2 at either the inlet or outlet of the chamber, depending on the setting of the stopcock. Because the metabolic equations require accurate and comparable inlet and outlet gas measurements, the analyzer is periodically switched between monitoring the inlet, reference gas (FiO2, FiCO2) and outlet gasses (FoO2, FoCO2) using the stopcock to select the gas sample source. This technique corrects for any small drift or absolute inaccuracy in the measurements; only the differential values are important (FiO2 – FoO2 and FoCO2 – FiCO2). Other considerations Theoretically, any flow rate through the chamber with a living animal inside will result in an O2 drop and a CO2 increase. However, the larger the inlet/outlet gas differentials are, the more accurate the results will be (within reason). The chamber serves as a mixing chamber where the inlet flow mixes with the animal’s (often tiny) exhalations, finally arriving at an equilibrium that can be measured at the chamber outlet. For best results, titrate the inlet flow against the outlet CO2 to achieve the desirable 0.2 – 1.0% CO2 differential. The O2 differential will concomitantly increase as well, allowing more accurate calculations. It is desirable to use a single set of gas sensors, such as in the Gemini monitor, to measure both reference gas as well as chamber outlet gas concentrations. This allows direct comparison between the inlet/outlet sample values. In the Figure, a 3-way stopcock is used to switch between monitoring reference or outlet gasses. This could easily be replaced with a 3-way electrically-operated solenoid valve to select the sample source by remote control. Most commercial dedicated metabolic monitor systems use this approach to multiplex a single set of sensors between sampling reference gas and the outlets of one or more metabolic chambers. It is important that measurement and reporting units are consistent. Published values of basic metabolic measurements may use ml/min, ml/min/g, or L/h/kg or any combination. Most values can be easily converted through judicious multiplication or division by 60 or 1000, depending on the desired units. Finally, while the basic measurements and calculations in open-circuit calorimetry are straightforward, there are many subtleties involved in producing successful measurements. The references below point out some of the difficulties as well as provide tips on getting good data. *Metabolic Calculations are attached as a separate pdf file References (see links below) Indirect Calorimetry: History,Technology, and Application. Mtaweh, Tuira, Floh and Parshuram, 2018 Simple equations for complex physiology: can we use VCO2 for calculating energy expenditure? Pierre Singer, 2016 Scaling of respiratory variables in mammals. Walter R. Stahl, 1967 | ||
Link al Producto:Referencias en PDF: Fuente CWE Incorporated | ||